
eDNA Analysis
Detection or identification of species from eDNA samples taken from waterbodies such as ponds, lakes, rivers and the ocean. See how our qPCR assays can assist with your next project.

Detection or identification of species from eDNA samples taken from waterbodies such as ponds, lakes, rivers and the ocean. See how our qPCR assays can assist with your next project.
Our eDNA Analysis service can identify if a particular target species or multiple specific target species are present or absent within a waterbody such as a pond, stream or river. eDNA surveys are non-invasive and highly sensitive with the ability to detect very low levels of species presence from a single sample. All results are provided as presence/absence. See our ‘species finder’ for a list of all the species are are able to detect. If you can’t find the species you’re looking for, we still may be able to help. Please check with us first to confirm that your chosen target species is possible to detect via eDNA in our laboratory.

David Kjaer / naturepl


Solvin Zankl / naturepl


Robert Thompson / naturepl

Michel Roggo / naturepl

Sinclair Stammers / naturepl

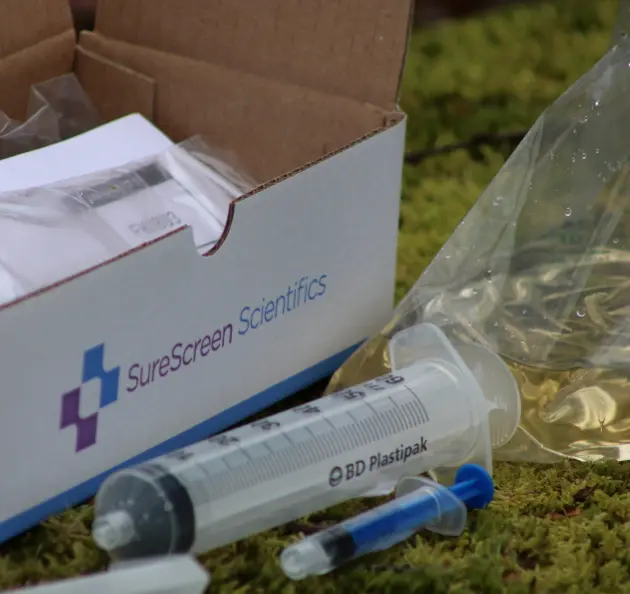
Our Filter eDNA Collection Kits have been specially designed and optimised to obtain the maximum amount of DNA from a water sample possible.
Filter eDNA Collection Kits (not GCN) £35 +VAT
By using filters, we can collect DNA from volumes up to 1L (over 10x the amount collected using the ethanol filled tubes for GCN eDNA). Only one Filter eDNA Collection Kit is needed per site, to detect up to four different target species. Mix and match to analyse up to four species including: white-clawed crayfish, signal crayfish, European eel, smooth newt, sea lamprey or any species tagged ‘eDNA’ in our species finder.
GCN eDNA Collection Kit £30 +VAT
Our GCN eDNA Kits have been carefully designed to make life in the field as easy as possible. All our great crested newt kits comply with the protocol outlined in WC1067. One kit is required for each pond, up to 1 hectare. We can also test for either common frog, common toad, alpine newt or smooth newt from the same sample.
Please send samples from either Filter eDNA Collection Kit (not GCN) or GCN eDNA Collection Kit with the relevant completed sample collection form. For advice on collecting quality samples see our detailed instructions or get in touch.
10
Working Days
£150 +VAT
price per sample
Standard Turnaround
5
Working Days
£250+VAT
price per sample
Fast Track
10
Working Days
£185 +VAT
price per sample
Standard Turnaround
5
Working Days
£285 +VAT
price per sample
Fast Track
10
Working Days
£220 +VAT
price per sample
Standard Turnaround
5
Working Days
£320 +VAT
price per sample
Fast Track
10
Working Days
£255 +VAT
price per sample
Standard Turnaround
5
Working Days
£355 +VAT
price per sample
Fast Track
There is no need to pre-book your analysis with SureScreen Scientifics, simply send your samples to us and we will email you to let you know when we have received them. The cost of analysis is invoiced to your account (for existing customers) and the results are made available when ready. New customers will need to pay for their analysis upfront before results are sent (for first order only).

We recommend using a local courier service such as DPD Local who will collect from you directly and deliver to us next working day. All you need to do is make sure your samples are packaged ready for collection. You will simply need to enter the approximate weight of the parcel (GCN kit is 0.5 kg / Filter kit is 0.3kg) and the collection address.
Important: Please do not arrange collections on a Friday as your samples won’t reach us until Monday meaning they will be stored in unknown conditions over the weekend so it is best to keep collections to Mon-Thurs for optimal sample quality.
Please send to:
SureScreen Scientifics Ltd, Morley Retreat, Church Lane, Morley, Derbyshire, DE7 6DE.
We also accept hand-delivered kits between 08.00 and 16.00 Monday to Friday, and by appointment 24/7.
There is no need to pre-book standard analysis with SureScreen Scientifics, simply send your samples to us and we will email you to let you know when we have received them.
IMPORTANT: Please call ahead to check availability if wishing to use our 5 working day service
The cost of analysis is invoiced to your account (for existing customers) and the results are made available when ready.
NB: New customers will need to pay for their analysis upfront before results are sent (for first order only)
Results for GCN eDNA surveys are only accepted by Natural England if the samples are collected between mid-April and late-June, however, we can analyse samples taken all year round for other applications.
Currently we suggest surveying between March and September when individuals are most active. Upon obtaining further data outside this period this may be revised at a later date.

Similar to GCN, adults emerge from hibernation on land from late February, returning to freshwater to breed. By October, most smooth newts will be back on land and preparing to hibernate.
The common frog is often found in ponds across the UK between February and October. We would therefore recommend sampling is best completed during this period
Due to seasonal weather conditions and changes in crayfish activity over the year, sample collection is best suited to the summer months where crayfish activity levels are higher.
Detectable all year round due to the presence of spores, it is recommended to avoid winter months when extreme weather conditions can cause a dilution of detectable DNA.
After spawning, European eels spend around 8 years in UK waters without before leaving for the Sargasso Sea, so we reccomend basing eDNA surveys around the fishing season.
The Atlantic salmon is found in rivers in Wales, Scotland and North and South West England, however, populations have been recently declining
Crusian carp and common carp are preset in UK waters all year round. Reccomended sampling time is based around warmer months where both species are more active.
For best results sample May-September when this species is most active.
Overwintering demon shrimp begin feeding in the spring and reproducing in April. With up to 3 generations produced each year, the breeding seasons finish in October and activity levels drop until the next spring.
Freshwater pearl mussels are present in water throughout the year; however, activity levels drop off over winter. During summer months, individuals are readily feeding and reproduction occurs within this period, greatly increasing the chances of detecting eDNA in the waterbody. This period is also best to avoid the high water flows of winter months.
This depends on the size of the site and the question that you are trying to answer. For a small isolated site such as a pond, small lake or isolated river site, one sample should be sufficient. For larger river systems and reservoirs, multiple samples should be taken from multiple sites to ensure the site is best represented by the sampling strategy. If you are struggling to determine how many samples to collect for a study site, then get in touch with us and we will be able to advise.
If you require a high level of scientific accuracy we recommend that you collect replicate samples from each site to ensure consistency and reliability in results.
Each sample can be analysed for up to four different eDNA target species currently offered by SureScreen. Once the DNA has been extracted from a sample it can then be analysed multiple times: i.e. for white-clawed crayfish, signal crayfish, marbled crayfish and the crayfish plague.
The assays used in the laboratory for the detection of each target species have by design been developed as species specific. This means that they will only detect and amplify DNA of the target species, thorough testing has been conducted to ensure that this is the case and that each of our assays for our eDNA services do not cross-amplify any other species.
With the crayfish plague and chytrid fungus being a large problem for native species in the UK at the moment, it is highly important that biosecurity measures are followed when collecting eDNA samples. We have designed a biosecurity friendly Filter eDNA Collection Kit which includes single use components and therefore reduces the risk of transferring material from site to site. However, it is also important that the end-user thoroughly cleans any additional equipment, wellies and clothes which they take to any site before moving onto a new site to reduce the risk of transferring any spores.
Anyone can sample! A license is only required if you are conducting a white-clawed crayfish survey additionally to the eDNA survey. If you are just simply taking a water sample and not intending to search for crayfish or disturb their habitat then you do not require a licence.
At the moment, this assay cannot be used on a legal basis to support planning and building applications. However, it can be a useful survey tool for the primary detection of populations as a complimentary survey technique.
This method is not currently approved by any approval body, however we are working towards this with the Environment Agency taking an assisting role with the validation of our method.
This varies for each species and should be designed around the life cycle/life history of your target species to coincide with periods in which they are most active. Samples can be taken outside of this window but may not provide highly reliable or repeatable results. Further to this the sample should be collected when the pond/river/stream is relatively calm, with little turbidity. Try to avoid sample collection from murky rivers/ponds or at sites just after large rainfall as the filter will soon clog and you will be unable to pass a sufficient volume of water through for analysis.
The filters are designed to process up to 1 litre of fluid. However, in the case of rivers and streams, due to turbidity and sediment load it may not always be possible to filter such a high volume. We recommend the filtration of at least 150ml with an ideal filtered volume of 400ml, the higher the filtered volume the increased chance of obtaining eDNA within your sample (if it is present within the sample site). If you are unable to filter such high volumes – don’t worry. Just make a note of the volume which was filtered on the sample collection form.
One kit is required for one pond up to approximately 1 hectare (2.5 acres). For ponds larger than this is it recommended to collect one kit per hectare. Generally, the most accepted way of collecting samples for a large pond of say 4 acres, is that two kits be used, one to collect 20 sub-samples around one half of the pond and the other kit to collect 20 sub-samples around the other half of the pond. These kits are analysed separately (and therefore charged at two kits and two analyses) as to not dilute the sample and give best chance to detect eDNA.
Small Streams & Rivers (less than 10m wide/less than 30cm deep)
Where possible try to avoid entering the water system and collect samples from either side of the bank. If it is necessary and safe to enter the water system, collect samples following a zig-zag pattern. Ensure that you sample in an upstream direction to avoid the collection of ancient sediment which may contain historical DNA from the target species. If you enter the watercourse, ensure that you implement full biosecurity protocols.
Large Rivers
For larger more difficult to access rivers it may be necessary to use multiple collection kits in order to ensure the reliability of species presence/absence. If you are unable to enter the watercourse as in the methods above then try where possible to collect samples from the edges, collecting a sub-sample every few metres in order to get a representative sample from the site.
If studying a large river system, it may be necessary to collect multiple samples at multiple points. For example, the collection of a sample every 500-1000m within a river. This is to ensure that you identify any populations which may be fragmented within the stream. Collecting multiple samples also enables the user to accurately indicate where a population may lie within the stream, as opposed to only collecting one sample and only knowing if the species is present or absent.
Key Advice
Results indicate that the DNA levels drop below detectable amounts no later than one month after all individuals have left the system. This means that there is minimal chance of detecting a population which has been absent for several months.
eDNA should not be used as a replacement for established/traditional survey methods. In its present form it is designed to be complimentary to these methods, as a primary survey technique which can enable the screening of large areas in a short amount of time with minimal training and licensing needs.
We recommend using a local courier service such as DPD Local who will collect from you directly and deliver to us next working day. All you need to do is make sure your samples are packaged ready for collection. You will simply need to enter the approximate weight of the parcel (GCN kit is 0.5 kg / Filter kit is 0.3kg) and the collection address. Booking online is quick and easy – just CLICK HERE to book your collection.
Important: Please do not arrange collections on a Friday as your samples won’t reach us until Monday meaning they will be stored in unknown conditions over the weekend so it is best to keep collections to Mon-Thurs for optimal sample quality.
Please send to:
SureScreen Scientifics Ltd, Morley Retreat, Church Lane, Morley, Derbyshire, DE7 6DE.
We also accept hand-delivered kits between 08.00 and 16.00 Monday to Friday, and by appointment 24/7.
There is no need to pre-book standard analysis with SureScreen Scientifics, simply send your samples to us and we will email you to let you know when we have received them.
*** Please call ahead to check availability if wishing to use our 5 working day service ***
The cost of analysis is invoiced to your account (for existing customers) and the results are made available when ready.
NB: New customers will need to pay for their analysis upfront before results are sent (for first order only)
Please note that due to the nature of the kits, we are unable to issue refunds once dispatched. However, we are more than happy to responsibly recycle any unused kits for you, including kit components such as gloves.
There are hundreds more species we can detect. Get in contact to see if the assay for your target species has already been developed, and if it has we’ll be able to detect it for you.
Note: For non routine targets we may need to buy the primers specifically for you, this may take a few days and may incur the cost price of the primers depending on the number of analyses required.